False Positive Results in Fetal Genetic Testing Peer Reviewed

Understanding Fake Negative in Prenatal Testing
1
Fetal Medicine Foundation of America, Icahn School of Medicine at Mt. Sinai, New York, NY 10029, Us
2
Department of Obstetrics & Gynecology, Icahn School of Medicine at Mt. Sinai, New York, NY 10029, USA
three
Department of Genomic Medicine, Changhua Christian Hospital, Changhua 50046, Taiwan
4
Department of Obstetrics and Gynecology, National Taiwan Academy Hospital, College of Medicine, Taipei 10041, Taiwan
5
Department of Biomedical Science, Dayeh University, Changhua 51591, Taiwan
6
Department of Medical Sciences, National Tsing Hua University, Hsinchu 30013, Taiwan
*
Writer to whom correspondence should be addressed.
†
All three authors contributed equally to this study.
Bookish Editor: Ritsuko Kimata Pooh
Received: 28 April 2021 / Revised: thirteen May 2021 / Accepted: 14 May 2021 / Published: 17 May 2021
Abstract
A simulated negative can happen in many kinds of medical tests, regardless of whether they are screening or diagnostic in nature. However, it inevitably poses serious concerns especially in a prenatal setting because its sequelae can mark the birth of an affected child across expectation. False negatives are non a new thing because of emerging new tests in the field of reproductive, specially prenatal, genetics but has occurred throughout the evolution of prenatal screening and diagnosis programs. In this newspaper we aim to discuss the bones differences between screening and diagnosis, the trade-offs and the choices, and also shed lite on the crucial points clinicians need to know and be aware of so that a quality service tin can exist provided in a coherent and sensible style to patients then that vital problems related to a fake negative result tin can be appropriately comprehended by all parties.
1. Introduction
The COVID pandemic has publicized some of the difficulties in the interpretation of screening tests that clinicians and statisticians have been debating for decades. Anyone paying attention has had a crash course in understanding that a "test" is not always perfect, in fact, it does non ever requite the right answer, and that there are trade-offs in the accurateness and reliability of results [1]. Those trained in the discipline of screening will, of form, recognize the utilization of some of its basic principles to explicate the current situation [2,3]. The majority of physicians and the full general public seem confused by the credible inability of the laboratories to give what was mostly causeless to exist simple yeah/no answers to very important questions.
The same principles employ to all forms of screening. In prenatal genetics, common examples include aneuploidy, specifically, Down syndrome using multiple methodologies, Mendelian carrier screening, and pre-eclampsia [iv,five,half dozen]. On the gynecology side of obstetrics and gynecology, screening for malignancies of the cervix, uterus, ovary, and breast have developed apace (simply are across the scope of this article) [7,8,9]. Before moving onto the nuances of emphasis that can be applied to how tests are designed and, every bit-chiefly, how cut-offs are arranged to favor either emphasis on sensitivity or accent on low fake positive rates, we will offset review some basics.
The offset issue is to distinguish betwixt screening and diagnostic tests. Simply put, screening tests only conform odds, and diagnostic tests are meant to requite a definitive answer (Table one). Historically, there have been between seven and ten criteria that have been felt necessary to consider earlier deciding to screen for a condition [2,three,10]. We aggrandize upon these and recategorize them to include issues of infrastructure, training, availability, acceptability, toll and political interference that earlier the COVID epidemic might have been taken for granted by those establishing guidelines for screening tests (Table 2).
Discussing these bug in any depth is beyond the telescopic of the present paper, merely the context in which screening and testing takes place can no longer exist taken for granted. In practice, not all tests being used, withal, follow these guidelines, which can lead to disproportionate expectations (either positive or negative), expenditures, and complications from follow-upwards testing that are likely "unnecessary". The goal of a screening program is to detect the maximum number of "affected" individuals for the least number screened simulated-positive. Specifically, a program is not judged by whether any particular patient's problem can be identified [1,2,3]. Where to put the cut-off points in balancing these two is, by definition, arbitrary, but must exist maintained to maximum efficiency.
Medicine, and particularly obstetrics, involves the repetitive utilize of both diagnostic and screening tests. Most patients and too many physicians do not understand the difference [1,2,iii]. Diagnostic tests are meant to give a definitive answer, may have risks, may exist expensive, and are only meant for patients at a risk high enough to warrant it. Conversely, screening tests are meant for "everyone", and their aim is to carve up a group with high-plenty risk to warrant diagnostic testing from those who practice not. They exercise not requite definitive answers [1,2,3]. How well they do their job is defined by metrics of sensitivity, specificity, positive, and negative predictive value. These principles of evaluation were introduced into clinical exercise in the 1970s by Galen and Gambino [1].
The performance characteristics establish the boundaries of a playing field and a scoring organization inside which tests compete for better ways to do things. Sensitivity and specificity every bit test properties are relatively well-known. Less ofttimes used are the criteria that summarize some of the critical trade-offs that clinicians face. One such trade-off is the relative number of true positive cases and imitation positive cases, which is reflected in the ratio of the ii: True positives and imitation positives are defined equally the Positive Likelihood Ratio (PLR). A second trade-off involves the relative number of faux negatives and true negatives, reflected in the ratio of the 2: Imitation negatives and true negatives are defined as the Negative Likelihood Ratio (NLR). Competitive approaches should have PLRs that are high and NLRs that are far less than one and demand to be "minimally partial".
Receiver operating feature curves are typically used to compare the efficacy of tests or combinations of tests by directly examining the trade-off between Truthful Positives (Sensitivity) and False Positives (1—Specificity). In situations where positive cases are very rare, so that the data are unbalanced, Precision-Call up Curves are more useful because they are not biased by the overwhelmingly large number of negative cases [11] and instead they focus more attention on the proportion of positive cases.
Clinicians are in the position of having to take all of this information and nowadays information technology in an understandable style to explain not just the prior risk of the patient, but also how that risk is changing over time using Bayesian principles [12]. In our feel, clinicians are more likely to meet value in a screening exam that changes a priori risk from 1/500 to 1/20 than one that changes it from i/100,000 to 1/500 even though the likelihood ratio of the erstwhile is improved 10 times more in the former than in the latter. An explanation for this would be that in the quondam, a alter in clinical behavior might occur whereas such change is less likely with the latter. Nosotros also previously reported that patients "all of a sudden" at risk (eastward.g., abnormal aneuploidy screening) had a higher "state of anxiety" over the situation than those with the aforementioned level of risk, but it was because of non-emergent risks such as advanced maternal historic period [13].
2. Choices
Given that, by definition, screening tests just adjust odds, there are options as to where to place the emphasis of the programme. In our general approach to genetic counseling, we routinely tell patients that in most circumstances, for those in the "centre 98%", it does not make a difference whether the patient has diagnostic testing for genetic abnormalities or not and everything will exist fine. The question that we believe all patients need to empathize is: "If yous're going to be wrong, which way would you rather be wrong? Would you rather accept a small-scale adventure (e.chiliad., a two% risk of having a baby with a serious problem vs. a 0.1–0.12% run a risk of having a complication from a procedure in experienced easily) considering you wanted to know that" [14,15,16]? We tell them that essentially the eye does not count.
The whole exercise then reduces to one question: "Tell me what you fear the virtually, so we tin reduce that at the expense of the other options". Unfortunately, encouraging patients to exist active at the dispensary level cannot be causeless to be representative beyond the board for at least three reasons. Showtime, screening might be treated every bit office of routine medical care not "requiring" whatsoever patient decision-making processes [17], thus undermining the possibility that informed consent and shared decision-making would exist part of the decision. Second, there may be a breakdown in shared decision-making owing to a various combination of class, racial-ethnic, or educational differences between genetic counselors and patients and the situation could create a collective fiction rather than shared controlling [18]. Third, ideological differences may create at least a suspicion of genetic reasoning and assay or outright hostility to it [19] or seek to create conditions in which genetic analysis assumes eugenic overtones [20,21].
The purpose of a screening program is not to maximize the correct diagnosis of whatsoever given patient. Rather it is to maximize the collective detection of bug for the to the lowest degree number of false positives. It can also exist thought of as mimicking the upstanding principle of proportionality, i.east., obtaining the most benefit for the least harm. A particularly good example was the utilize of maternal serum blastoff-fetoprotein (MSAFP) for neural tube defects (NTDs). In the 1970s to the mid-1980s, the standard cut-off of 2.5 multiples of the median identified most 90% of NTDs for about a five% imitation positive charge per unit. Moving the cut-off to the right would increment the positive predictive value at the expense of sensitivity. Moving information technology to the left would increase the sensitivity only besides create many more simulated positives (Figure 1). At that place is no absolute clarity as to where on the continuum the optimal cut-off signal should be.
Whether to maximize sensitivity or keep the screen positive number to a minimum involves non but mathematical analysis only clinical and upstanding value judgements [2,3] (Effigy two). In that location are many components in the controlling process for patients and couples as to whether they want diagnostic testing.
Given the upstanding, religious and politically charged nature of prenatal diagnosis and its relationship to the abortion event, it is not surprising that at that place are vast differences in the utilization of such technologies by regions of the world. Within big countries such as the United States, variations occur as a consequence of political affiliation, religious beliefs, social class and instruction, as well as multiple other personal and traditional "sociological" variables.
Policy makers accept to make decisions near where to put cutting-off points. Setting policies may be decided past the program directors, government officials, or insurance companies. They may exist uniform throughout a state or vary enormously even in the same environment. Alpha-fetoprotein (AFP) diagnosis from amniotic fluid was developed in 1972. At that fourth dimension, the recurrence risk in the United Kingdom for a couple with i prior NTD was about three–5% and, with 2 prior NTDs it was nearly 10%. Such patients were directly referred for amniocentesis even though its risks were idea to be nearly ii%, and because ultrasound was non-existent [22]. While recurrence risks were much higher than the primary incidence, 95% of all NTDs occurred in couples with no prior or family unit history [23,24]. The overall chief incidence of NTDs in Scotland was almost ane/400. Given the high risk of procedures at the time, a screening arroyo was the just realistic public wellness measure to detect a highly pregnant or highly prevalent disorder. MSAFP was first introduced in 1973 every bit a screening examination to make up one's mind who, amid the supposedly depression risk grouping, was at high risk [25].
The MSAFP cutting-off was determined based upon conversations between the laboratory and the obstetrics department in Edinburgh to model what cut-off point was required to produce, by identifying and ranking the highest risk patients, the maximum number of "at risk" patients that could be accommodated past the clinical service at the hospital. Having the resource available is a relevant and rational contextual element is purely rational only assuming that the political process volition operate in a way that is driven but by adventure tin no longer be taken for granted. Extending this argument, one tin no longer presume that tests will not be reserved for those of higher condition, or, on the other side, that tests will not be routine for those with lower condition. At the most general level, neither micro-contexts nor macro-contexts [26] may exist assumed to be immune from political influence.
Implementation has besides been problematic. In 1988 nosotros reported enormous variation in MSAFP screening in the Detroit area such that the same specimen could be interpreted by laboratories across the full spectrum from being very high risk to very depression risk. Likewise, some laboratories stated the need for certain parameters to be known (such every bit maternal weight and ethnicity), merely and so did not insist that they be provided, or even include them in their assay [27]. It took decades to coalesce on quality control measures to ensure better standardization of the accuracy of measurements and the relationship between the raw measurement and its clinical interpretation. In the 1970s, it was common belief that laboratories in unlike cities needed to have their ain reference ranges for MSAFP because the populations were "different" [28]. Eventually, it was determined that the discordance was, to an exceptionally big extent, a function of different laboratory methodologies. When standardized across the country the differences between cities completely disappeared. Racial and ethnic differences still remained, simply they alsodid not vary across cities.
In the mid-1980s, equally MSAFP screening moved from just NTDs (high MSAFP) to adding Down syndrome and Trisomy 18 (depression MSAFP), assay changes were required to increase the accuracy of depression-level measurements [29]. I effect was a distribution shift such that the percentage of cases with high MSAFPs fell from well-nigh 5% to ii.5%. Given that the bulk of NTD cases had MSAFP values over 4.0 multiple of the median (MoM), the alter resulted in very few additional fake negatives and simultaneously improved the positive predictive value of those cases at take chances [30]. Increasing the quality of ultrasound applied science and clinical expertise likewise mitigated the risks of missed MSAFP screens [31].
Also, equally MSAFP expanded to double (MSAFP + full human chorionic gonadotropin (hCG), then with some centers substituting costless β hCG for total), triple (adding estriol), and somewhen quad(adding inhibin), at that place were many arguments over the all-time combination with nigh arguing about estriol [32]. Arguments between the "double" vs. the "triple" supporters reached almost religious fervor. (Nosotros belonged to the "double" religion). Ultimately, assay showed that whereas variables such MSAFP and the hCGs had stable coefficients of variation (COV) at around 5–vii%, the estriol analysis was much less accurate [32]. Some laboratories reported a similar v–seven% COV and demonstrated that estriol added value. However, for many laboratories, the COV was as much as 30%, rendering estriol useless or worse [32].
With the introduction of nuchal translucency (NT) measurements, the COV problem became considerably worse. In order to command for highly variable quality and accuracy, the Fetal Medicine Foundation in London developed training and screening reviews and certifications and showed increasing quality globe-wide [33]. After the Nuchal Translucency Quality Review (NTQR) program of the Social club for Maternal Fetal Medicine in America introduced preparation programs in the United States. We reported that the Fetal Medicine Foundation program seemed to generate higher quality command than NTQR which atomic number 82 to higher sensitivities and specificities [34,35]. We adult an approach to "handicap" less proficient providers by modulating how much those measurements counted in the algorithm [36]. Information technology worked but never made it into the mainstream equally the concurrent development of prison cell costless fetal DNA came into practice whose protocols almost eliminated the use of NT measurements for Down syndrome screening in the practice mural of prenatal diagnosis and obstetrics.
The two situations above (COV of estriol and NT quality) illustrate the conundrum of trying to amend the statistical performance of screening past insisting upon quality controls. It can be difficult in the laboratory; it is much harder for more subjective variables, e.g., ultrasound NT measurements, with multiple clinicians involved [33,34,35,36]. Each provider has their own strengths and weaknesses, and there is well known variability amid providers [37]. These types of situations emphasize further problems of gradient between absolute conformity of all care and widespread individuality that can drag some providers merely reveal weaknesses at the same time. The former elevates weaker providers but inhibits excellence at the top finish.
Once Down syndrome became the master focus of screening, multiple generations of improvements accept occurred. The shifts from MSAFP to double, triple, and quadruple screening in the second trimester and NT, costless β hCG, pregnancy associated plasma protein A (PAPP-A), combined screening, cell costless fetal Dna (cffDNA) in diverse forms in the offset trimester take all had the underpinnings of simultaneously improving both sensitivity and specificity [38,39,40]. Ironically, however, the improved screening for Down's syndrome has come at the cost of increased failures to notice other problems that could accept been found by using loftier quality ultrasounds and diagnostic procedures such every bit chorionic villus sampling with improved laboratory tests such as microarrays that can discover, especially in younger patients, 10 times the abnormalities that can exist washed by cffDNA [xiv,xv,sixteen].
Changes in the perceived efficacy of tests can have wide ranging furnishings upon the practice of medicine and club. On the positive side, improvements in Mendelian Screening have allowed the identification of at-chance couples to occur Earlier they have experienced the affect of an affected child. As well, virtually fetal cardiac anomalies can now be detected prenatally allowing births to be moved to centers capable of advanced care rather than having to have panic neonatal transfers or no transfers at all [41,42]. Unfortunately, the rate of incorporation of new approaches is highly variable amongst industries. Medicine has historically been on the slower stop of the spectrum and obstetrics on the slower end of medicine [43]. NT screening is a good example. It was adopted much faster in the UK and Europe than in the USA [37,43]. Likewise, there is a pattern in which there is first the development of new technologies followed by diffusion out to the community. As new methods expand, the utilization increases, only complications can increase rapidly until at that place is time for community agreement and education [44].
The sociological and societal implications of such agreement have dramatic implications for the practise of medicine and the health of the population. Due to the increased "screening" for Down syndrome at that place has been diminished utilization of procedures that we have described as an epidemic of MISSED abnormalities because of cffDNA. The public health effect has meant more unintended babies with serious disorders that, aslope other issues, accept expensive care requirements [14,15,16].
Fifty-fifty getting past technical, fiscal, implementation issues, and quality controls, there are still philosophical issues that bulldoze the determination of a cut-off indicate. The more than serious the disorder, the more probable that any typical patient'south "tolerance" for missing a example would exist very low [45,46]. This would create an impetus to put the cutting-off indicate "to the left" on the distribution curve which would maximize sensitivity at the expense of increased simulated positives. Likewise, with disorders with a very low incidence or those in which morbidity and bloodshed might exist moderate, it would exist reasonable to motion the cut-off to the right to minimize the number of patients undergoing follow-up diagnostic procedures [i,2,3] (Figure 2).
Nosotros have previously modeled the detection of genetic disorders comparing those identifiable by cffDNA versus diagnostic testing using chorionic villus sampling or amniocentesis with enhanced genetic testing using array comparative genomic hybridization (aCGH or microarrays) [fourteen,xv,sixteen]. We reported that the yield of detected abnormalities was about 10 times greater using the diagnostic testing vs. screening. While this is to some caste an apples vs. oranges comparison (screening vs. diagnostic), it does highlight the merchandise-off of simplicity and lower cost versus comprehensiveness and greater expense for the detection and management of rare but pregnant outcomes. More directly examples within the screening world would exist karyotype vs. microarray, small ethnically derived Mendelian screening panels vs. larger pan-ethnic panels, and Pap smears and mammograms vs. BRCA and Lynch syndrome molecular panels [seven,8,9].
Some prenatal diagnosis centers are known for their procedure acumen. Patients who want "definitive" answers tend to get to such centers, oft directed past referring physicians sensitive to their patient's desires [14]. Conversely, other centers are known for a limited utilise of diagnostic procedures. To a degree, everyone "votes with their feet" as to where they go. A continuing challenge is assuring that patients have sufficient access and understanding to make informed "foot" (certainly autonomous) choices.
For some parameters, such every bit advanced maternal historic period, in that location are at least de facto standards every bit to what the borderline is. In the United states, advanced maternal age was 40 in the early 1970s, then vicious to 38, and has been 35 for over 3 decades [3]. However, screening has used the cut-off as a starting point. For methods such as MSAFP, multiple markers and ultrasounds in the second trimester, and combined screening and nuchal translucencies in the first trimester, statistically employ a "likelihood ratio" to multiply (×) the screening result with the a priori chance of achieving a take a chance which is and then compared to the a priori risk [34,36]. The situation with cffDNA is more than complicated as some laboratories use an absolute, (i.due east., we see it, or we do not), and others base their risk prediction estimate on the groundwork risk every bit defined by maternal age. Some vary their risk certainty by the fetal fraction of the specimens, and others practise not [47]. These variations in style occur as physicians and clinics adjust to the needs, preferences and cultures of the populations they serve. The systemic claiming is to continually update both training and the diffusion of more than effective technologies.
cffDNA testing was originally publicized past its developers to be a diagnostic test. The acronym NIPD for noninvasive prenatal diagnosis was commonly used. However, it soon became articulate that "NIPD" was only a screening test based upon adding no matter which methodology was chosen [48].
Fetal cell testing was stopped when cffDNA was developed. Recently, some researchers have tried to revive the cell-based non-invasive prenatal testing (cbNIPT). In that location are theoretical advantages of cells vs. cffDNA. Prison cell-based methods would give a more directly confirmation of fetal vs. maternal DNA and could take faster whole-genome applications. If and then, the cell-based testing could be the solution to creating a diagnostic rather than a screening exam and thus be capable of reducing fake negatives inherent in cffDNA prenatal testing.
Some early cbNIPT studies (1970s and 1980s) were based upon capturing trophoblasts, which intuitively cannot solve the limitation of fetoplacental mosaicism [49,l]. In the 1990s and early 2000s, most approaches, including the National Plant of Child Wellness's "NIFTY written report" used nucleated erythrocytes, but the technologies were not robust plenty [51]. Newer methods using fetal nucleated red blood cells (fnRBCs) might exist able to solve the limitation of fetoplacental mosaicism which is inherent in both trophoblast-based cbNIPT and cffDNA testing. However, the possible revival of cbNIPT, even with the utility of fnRBCs instead of trophoblasts as the diagnostic target, will be congenital upon the prerequisite to solve the long-existing limitations of the technologies themselves: questionable reproducibility, consistency, scalability, and fifty-fifty reliability should be improved first. Automation past incorporating artificial intelligence (AI) to reduce manual work may exist a way to assist, and the progress in the highly similar field of circulating tumor cells (CTCs) tin can be referenced [52]. Much more work, including large-scale validation studies, is needed to verify its feasibility [53,54]. Supposing such efforts past capturing fnRBCs materialize, the trouble of true fetal mosaicism is however beyond the scope of such engineering.
Nosotros raise this issue to highlight the next generation "tug of war" between screening versus diagnostic approaches that we take previously addressed between cffDNA and microarrays [14,fifteen,16]. Well-informed genetic counseling will exist critical before utilizing any emerging new genetic testing, either screening or diagnostic. Considerable evidence suggests that the vast majority of obstetricians world-broad are non well-versed in the new technologies [14,15,16,47,55]. The more reliable a test is, the less dependent wellness care delivery will be on resources that do non exist for a very high-level clinical interpretation of information.
iii. Conclusions
A diagnostic test is meant to give a definitive answer. Screening tests but alter odds. In that location are many assumptions and decisions that have to be fabricated arbitrarily by the program directors that profoundly influence the outputs and tin can be swayed towards one extreme (maximizing sensitivity) or the other (minimizing screen positives). Fifty-fifty minor shifts in screening plan philosophy and practice tin can accept enormous public wellness implications.
About programs practice not publicly report these underlying decisions, or how their operations conform or vary from other laboratories in their expanse. Nigh programs likewise do not declare their endpoints in situations in which outcomes are not clear-cut and how such cease indicate dubiety colors their predictive values. In that location is no likelihood that in that location will be whatsoever uniformly agreed-upon strategy for such decisions, but clinicians should at least be enlightened of these issues and how they bear on the performance of all screening tests. Understanding the scientific and sociological contexts of imitation negative results, in add-on to false positive results, will be very important when clinicians are interpreting the results of prenatal screening and diagnostic tests and thereby can offer counseling to their patients with acceptable and decent quality. The misconception of confusing a screening exam with a diagnostic one volition bring negative impacts to the overall obstetric do and the populations they serve, as nosotros take seen in the recent history when cffDNA-based NIPT was introduced since 2011 [fourteen,xv,16].
Author Contributions
M.I.E., K.C. and D.W.B.; writing—original draft preparation, Thou.I.E., Thou.C. and D.W.B.; writing—review and editing, G.I.East., M.C. and D.Due west.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded past Changhua Christian Hospital, grant number 109-CCH-PRJ-117.
Conflicts of Interest
The authors declare no conflict of involvement.
References
- Galen, R.Southward.; Gambino, S.R. Across Normality: The Predictive Value and Efficiency of Medical Diagnoses; Wiley: New York, NY, USA, 1975. [Google Scholar]
- Evans, I.M.; Krivchenia, X.; Wapner, R.; Depp, R. Principles of Screening. Clin. Obstet. Gynecol. 2002, 45, 657–660. [Google Scholar] [CrossRef]
- Evans, M.I.; Eden, R.D.; Britt, D.W.; Evans, S.M.; Schifrin, B.S. Re-conceptualizing fetal monitoring. Eur. J. Gynecol. Obstet. 2019, one, 10–17. [Google Scholar]
- Haque, I.S.; Lazarin, G.A.; Kang, H.P.; Evans, E.A.; Goldberg, J.D.; Wapner, R.J. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA 2016, 316, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, D.Fifty.; Wright, D.; Poon, L.C.; O'Gorman, N.; Syngelaki, A.; de Paco Matallana, C.; Akolekar, R.; Cicero, South.; Janga, D.; Singh, Chiliad.; et al. Aspirin versus placebo in pregnancies at loftier gamble for preterm preeclampsia. NEJM 2017, 377, 613–622. [Google Scholar] [CrossRef]
- Hashiloni-Dolev, Y.; November-Klaiman, T.; Rza, A. Pandora's pregnancy: NIPT, CMA, and genetic sequencing—A new ear for prenatal genetic testing. Prenat. Diagn. 2019, 39, 859–865. [Google Scholar] [CrossRef]
- Committee on Exercise Bulletins-Gynecology. Exercise Message number 179: Chest Cancer risk cess and screening in average risk women. Obstet. Gynecol. 2017, 130, e1–e16. [Google Scholar] [CrossRef] [PubMed]
- Pellat, A.; Netter, J.; Perkins, K.; Cohen, R.; Coulet, F.; Parc, Y.; Svrcek, M.; Duval, A.; André, T. Lynch syndrome: What is new? Bull. Cancer 2019, 106, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Staples, J.N.; Duska, L.R. Cancer screening and prevention highlights in gynecologic cancer. Obstet. Gynecol. Clin. North. Am. 2019, 46, 19–36. [Google Scholar] [CrossRef]
- Wilson, J.M.G.; Jungner, G. Principles and Practice of Screening for Affliction; World Wellness Organization: Geneva, Switzerland, 1968. [Google Scholar]
- Saito, T.; Rehmsmeier, M. The precision call up plot is more informative than the ROC plot when evaluating classifiers on imbalanced datasets. PLoS ONE 2015, x, e0118432. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Wilson, R.B. Bayesian analysis and hazard assessment in genetic counseling and testing. J. Med. Diagn. 2004, 6, 1–ix. [Google Scholar] [CrossRef]
- Evans, Chiliad.I.; Bottoms, S.F.; Carducci, T.; Grant, J.; Belsky, R.Fifty.; Solyom, A.E.; Quigg, One thousand.H.; LaFerla, J.J. Determinants of altered anxiety following abnormal maternal serum alpha-fetoprotein screening. Am. J. Obstet. Gynecol. 1988, 159, 1501–1504. [Google Scholar] [CrossRef]
- Evans, G.I.; Wapner, R.J.; Berkowitz, R.L. Noninvasive prenatal screening or advanced diagnostic testing: Caveat emptor. Am. J. Obstet. Gynecol. 2016, 215, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Evans, Thousand.I.; Andriole, S.; Curtis, J.; Evans, S.M.; Kessler, A.A.; Rubenstein, A.F. The epidemic of abnormal copy number variant cases missed because of reliance upon noninvasive prenatal screening. Prenat. Diagn. 2018, 38, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.I.; Evans, S.Thousand.; Bennett, T.A.; Wapner, R.J. The price of abandoning diagnostic testing for cell-free fetal DNA screening. Prenat. Diagn. 2018, 38, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Santalahti, P.; Hemminki, E.; Aro, A.R.; Helenius, H.; Ryynänen, M. Participation in prenatal screening tests and intentions concerning selective termination in Finnish medical care. Fetal. Diagn. Ther. 1999, 14, 71–79. [Google Scholar] [CrossRef]
- Press, Northward.; Browner, C.H. Characteristics of women who turn down an offering of prenatal diagnosis: Data from the California maternal serum blastoff fetoprotein blood test experience. Am. J. Med. Genet. 1998, 78, 433–445. [Google Scholar] [CrossRef]
- Matthew, D.B. Two threats to precision medical equity. Ethn. Dis. 2019, 29, 629–640. [Google Scholar] [CrossRef]
- Fletcher, J. The long view: How genetic discoveries will assistance health care reform. J. Women's Health 1998, vii, 817–823. [Google Scholar] [CrossRef]
- Duster, T. Backdoor to Eugenics, 2nd ed.; Routledge: New York, NY, The states, 2003. [Google Scholar]
- Wald, N.; Cuckle, H. Amniotic fluid blastoff-fetoprotein measurement in antenatal diagnosis of anencephaly and open up spina bifida in early pregnancy. Lancet 1979, i, 685–688. [Google Scholar] [CrossRef]
- Gastel, B.; Haddow, J.E.; Fletcher, J.C.; Neale, A. Maternal Serum Alpha-Fetoprotein: Issues in the Prenatal Screening and Diagnosis of Neural Tube Defects; U.Southward. Government Printing Role: Washington, DC, USA, 1980.
- Lupo, P.J.; Agopian, A.J.; Castillo, H.; Castillo, J.; Clayton, G.H.; Dosa, North.P.; Hopson, B.; Joseph, D.B.; Rocque, B.Grand.; Walker, Due west.O.; et al. Genetic epidemiology of neural tube defects. J. Pediatr. Rehabil. Med. 2017, 10, 189–194. [Google Scholar] [CrossRef]
- Brock, D.J.H.; Bolton, A.E.; Monaghan, J.M. Prenatal diagnosis of anencephaly through maternal serum blastoff-fetoprotein measurements. Lancet 1973, 2, 923–924. [Google Scholar] [CrossRef]
- Bronfenbrenner, U. Contexts of kid rearing: Issues and prospects. Am. Psychol. 1979, 34, 844–850. [Google Scholar] [CrossRef]
- Evans, M.I.; Belsky, R.L.; Greb, A.Eastward.; Dvorin, Eastward.; Drugan, A. Wide variation in maternal serum alpha-fetoprotein (MSAFP) reports in 1 metropolitan area: Concerns for the quality of prenatal testing. Obstet. Gynecol. 1988, 72, 342–345. [Google Scholar]
- Braoudakis, One thousand.; Marguerat, P.; Marazzi, A.; Gutzwiller, F. Interlaboratory variation in the prenatal screening for neural tube endmost defects. Soz. Praventivmed. 1984, 29, 207–208. [Google Scholar] [CrossRef]
- Macri, J.N.; Kasturi, R.V.; Krantz, D.A.; Hu, One thousand.One thousand. Maternal serum blastoff-fetoprotein screening: Ii. Pitfalls in low volume decentralized laboratory performance. Am. J. Obstet. Gynecol. 1987, 156, 533–535. [Google Scholar] [CrossRef]
- Reichler, A.; Hume, R.F., Jr.; Drugan, A.; Bardicef, M.; Isada, N.B.; Johnson, Grand.P.; Evans, M.I. Risk of anomalies every bit a office of level of elevated maternal serum blastoff-fetoprotein. Am. J. Obstet. Gynecol. 1994, 171, 1052–1055. [Google Scholar] [CrossRef]
- Schell, D.L.; Drugan, A.; Brindley, B.A.; Zador, I.E.; Johnson, Thousand.P.; Schwartz, D.B.; Evans, Grand.I. Combined ultrasonography and amniocentesis for significant women with elevated maternal serum alpha-fetoprotein: Revising the run a risk estimate. J. Reprod. Med. 1990, 35, 543–546. [Google Scholar]
- Evans, Thou.I.; Hallahan, T.W.; Krantz, D.; Galen, R.South. Meta-Analysis of outset trimester Down syndrome screening studies: Free beta hCG significantly outperforms intact hCG in a multi-marking protocol. Am. J. Obstet. Gynecol. 2007, 196, 198–205. [Google Scholar] [CrossRef]
- Nicolaides, K.H. Turning the pyramid of prenatal care. Fetal. Diagn. Ther. 2011, 29, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.I.; Krantz, D.A.; Hallahan, T.Westward.; Sherwin, J.E. Undermeasurement of nuchal translucencies: Implications for screening. Obstet. Gynecol. 2010, 116, 815–818. [Google Scholar] [CrossRef]
- Evans, M.I.; Krantz, D.; Hallahan, T.; Sherwin, J. Impact of nuchal translucency credentialing by the FMF, the NTQR or both on screening distributions and functioning. Ultrasound Obstet. Gynecol. 2012, 39, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.I.; Cuckle, H.South. Functioning Adjusted Risks: A method to amend the quality of algorithm performance while allowing all to play. Prenat. Diagn. 2011, 31, 797–801. [Google Scholar] [CrossRef]
- Evans, M.I. Overcoming Militant Mediocrity. Am. J. Obstet. Gynecol. 2008, 198, 656–661. [Google Scholar] [CrossRef]
- Evans, M.I.; Sonek, J.D.; Hallahan, T.W.; Krantz, D.A. Cell-gratis fetal Dna screening in the U.s.: A toll analysis of screening strategies. Ultrasound Obstet. Gynecol. 2015, 45, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Van der Meij, Thou.R.Grand.; Sistermans, E.A.; Macville, M.V.Eastward.; Stevens, S.J.C.; Bax, C.J.; Bekker, M.N.; Bilardo, C.M.; Boon, East.M.J.; Boter, Yard.; Diderich, G.East.M.; et al. TRIDENT 2: National implementation of genome wide non invasive prenatal testing equally a get-go tier screening test in kingdom of the netherlands. Am. J. Hum. Genet. 2019, 105, 1091–1101. [Google Scholar] [CrossRef]
- Bunnik, Due east.Thousand.; Kuipers, A.Thousand.; Galjaard, R.J.H.; de Beaufort, I.D. Should pregnant women be charged for non-invasive prenatal screening? Implications for reproductive autonomy and equal access. J. Med. Ethics 2020, 46, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Britt, D.W. Psychosocial issues in Prenatal Diagnosis. In Prenatal Diagnosis; Evans, M.I., Johnson, Thousand.P., Yaron, Y., Drugan, A., Eds.; McGraw Hill Professional person: New York, NY, USA, 2006; pp. 701–706. [Google Scholar]
- Donofrio, 1000.T. Predicting the future: Delivery room planning of congenital heart affliction diagnosed by fetal echocardiography. Am. J. Perinatol. 2018, 35, 549–552. [Google Scholar] [CrossRef]
- Evans, I.M.; Hanft, R. The Introduction of New Technologies; ACOG Clinical Seminars, ACOG: Washington, DC, USA, 1997; p. 3. [Google Scholar]
- Cohen, A.B.; Hanft, R.South. Engineering in American Health Care: Policy Directions for Effective Evaluation and Management; Academy of Michigan Press: Ann Arbor, MI, USA, 2004; p. 480. [Google Scholar]
- Britt, D.W.; Risinger, S.T.; Miller, V.; Mans, Yard.K.; Krivchenia, E.Fifty.; Evans, Yard.I. Determinants of parental decisions after prenatal diagnosis of Down syndrome: Bringing in context. Am. J. Med. Genet. 2000, 93, 410–419. [Google Scholar] [CrossRef]
- Britt, D.W.; Risinger, Southward.T.; Mans, M.; Evans, M.I. Devastation and relief: Conflicting meanings in discovering fetal anomalies. Ultrasound Obstet. Gynecol. 2002, twenty, 1–5. [Google Scholar] [CrossRef]
- Hui, L.; Bianchi, D.West. Fetal fraction and noninvasive prenatal testing: What clinicians need to know. Prenat. Diagn. 2020, xl, 155–163. [Google Scholar] [CrossRef]
- Che, H.; Villela, D.; Dimitriadou, East.; Mellote, C.; Brison, North.; Neofytou, One thousand.; Bogaert, M.Five.D.; Tsuiko, O.; Devriendt, Grand.; Legius, E.; et al. Non-invasive prenatal diagnosis by genome-broad haplotyping of prison cell-costless plasma DNA. Genet. Med. 2020, 22, 962–973. [Google Scholar] [CrossRef]
- Vossaert, L.; Wang, Q.; Salman, R.; McCombs, A.K.; Patel, Five.; Qu, C.; Mancini, Grand.A.; Edwards, D.P.; Malovannaya, A.; Liu, P.; et al. Validation study for single circulating trophoblast genetic testing every bit a form of noninvasive prenatal diagnosis. Am. J. Hum. Genet. 2019, 105, 1262–1273. [Google Scholar] [CrossRef]
- Ravn, K.; Singh, R.; Hatt, Fifty.; Kølvraa, Thou.; Schelde, P.; Vogel, I.; Uldbjerg, N.; Hindkjær, J. The number of circulating fetal extravillous trophoblasts varies from gestational week 6 to xx. Reprod. Sci. 2020, 27, 2170–2174. [Google Scholar] [CrossRef]
- Bianchi, D.West.; Simpson, J.Fifty.; Jackson, L.G.; Elias, L.G.; Holzgreve, W.; Evans, 1000.I.; Dukes, One thousand.A.; Sullivan, 50.M.; Klinger, G.W.; Bischoff, F.Z.; et al. Fetal gender and aneuploidy detection using fetal cells in maternal claret: Analysis of Peachy I data. National Institute of Child Health and Evolution Fetal Cell Isolation Study. Prenat. Diagn. 2002, 22, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Jou, H.J.; Chou, L.Y.; Chang, Westward.C.; Ho, H.C.; Zhang, W.T.; Ling, P.Y.; Tsai, K.H.; Chen, T.H.; Chen, Due south.H.; Lo, P.H.; et al. A novel automatic platform based on nanostructured microfluidic bit for isolating and identification of circulating tumor cells. Micromachines 2021, 12, 473. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Due east.; Ma, G.C.; Jou, H.J.; Lin, Due west.H.; Lee, D.J.; Lin, Y.S.; Ginsberg, North.A.; Chen, H.F.; Chang, F.One thousand.C.; Chen, Due west. Noninvasive prenatal diagnosis of fetal aneuploidy past circulating fetal nucleated red blood cells and extravillous trophoblasts using silicon-based nanostructured microfluidics. Mol. Cytogenet. 2017, ii, 44. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.C.; Lin, W.H.; Huang, C.Due east.; Chang, T.Y.; Liu, C.Y.; Yang, Y.J.; Lee, Yard.H.; Wu, W.J.; Chang, Y.S.; Chen, M. A silicon-based coral-like nanostructured microfluidics to isolate rare cells in human circulation: Validation by SK-BR-3 cancer jail cell line and its utility in fetal nucleated red blood cells. Micromachines 2019, 10, 132. [Google Scholar] [CrossRef]
- Dondorp, Due west.; de Wert, Yard.; Bombard, Y.; Bianchi, D.Westward.; Bergmann, C.; Borry, P.; Chitty, L.S.; Fellmann, F.; Forzano, F.; Hall, A.; et al. Non-invasive prenatal testing for aneuploidy and beyond: Challenges of responsible innovation in prenatal screening. Eur. J. Hum. Genet. 2015, 23, 1438–1450. [Google Scholar] [CrossRef]
Figure ane. Typical cut-off of 2.5 MOM identifies virtually ninety% for a 5% fake positive rate. Moving the cut-off to iv MOM would significantly increase the positive predictive value in an abnormal, but would too result in many fake negatives. Moving information technology far to the left (about 0.6 MOM) would increase the sensitivity by almost 100% only at the price of having a screen positive rate of nearly 70%.
Effigy one. Typical cut-off of 2.5 MOM identifies about xc% for a 5% false positive rate. Moving the cut-off to iv MOM would significantly increase the positive predictive value in an abnormal, but would also result in many simulated negatives. Moving information technology far to the left (about 0.6 MOM) would increase the sensitivity past about 100% just at the toll of having a screen positive rate of nearly lxx%.
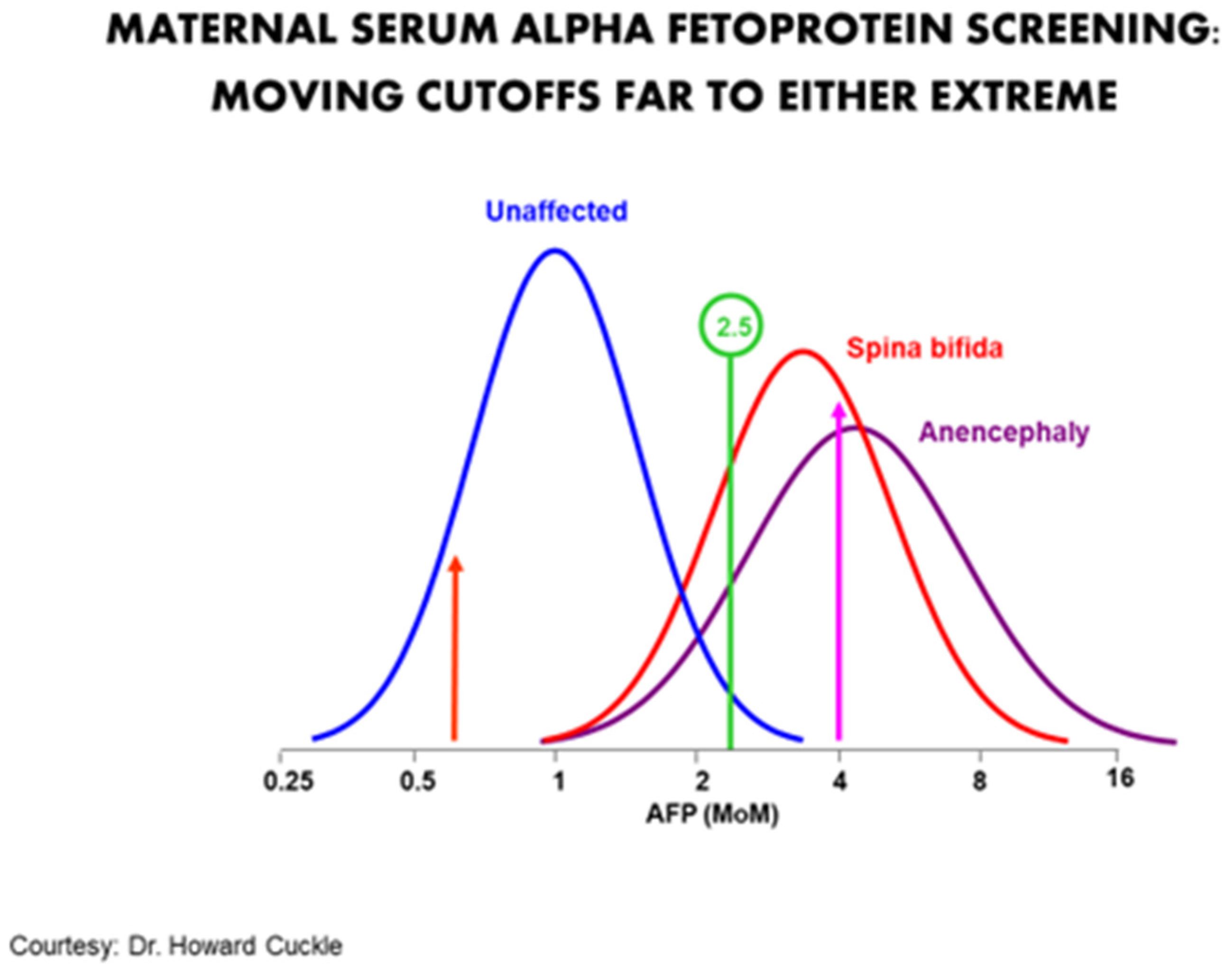
Figure ii. Choices for cut-off points.
Effigy two. Choices for cutting-off points.
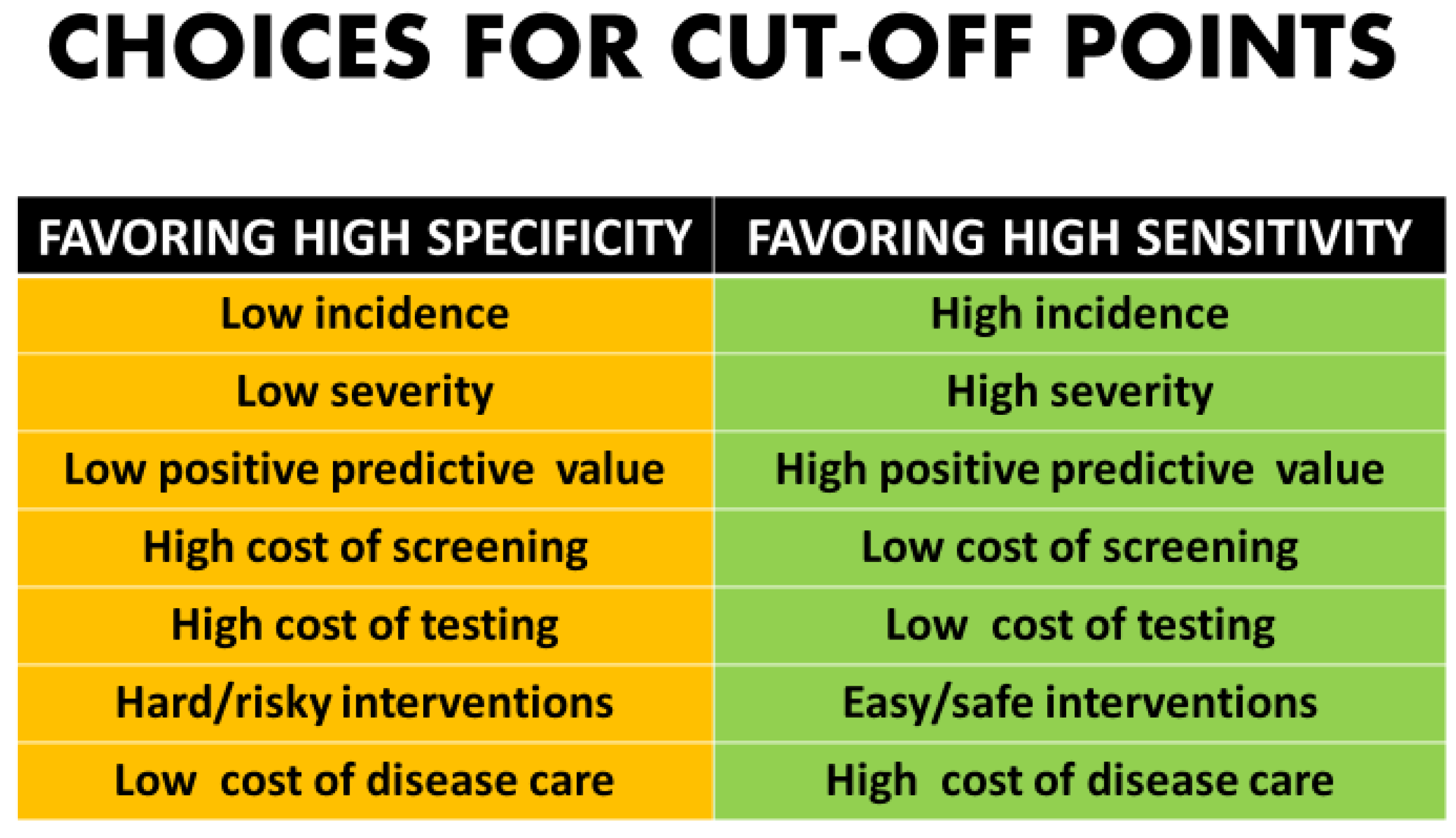
Table 1. Diagnostic vs. screening tests.
Table 1. Diagnostic vs. screening tests.
| Screening Tests | Diagnostic Tests |
|---|---|
| Meant for everyone | Only done on "at risk" patients |
| Only adjust odds and practice not give a definitive answer | Meant to requite a definitive answer |
| Tests typically have little gamble | Tests may have some procedural risks |
| Typically less expensive | Typically more expensive |
Table 2. Criteria for effective screening and testing programs.
Tabular array 2. Criteria for effective screening and testing programs.
| Disease | Screening | Testing | Intervention |
|---|---|---|---|
| High enough incidence | Proficient operation metrics | Good performance metrics | Benign intervention possible |
| Availability and affordability of screening | Availability and affordability of testing | Availability and affordability of intervention(s) at different levels | |
| Acceptability of screening | Acceptability of testing | Acceptability of intervention(due south) at unlike levels | |
| Impairing or fatal | Capacity for follow-upwards and feedback | Capacity for follow-up and feedback | Benefits outweigh risks |
| Acceptable political support and coordination for public health | Acceptable political support and coordination for screening | Adequate political support and coordination for testing | Adequate political support and coordination for interventions |
| Publisher's Notation: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 past the authors. Licensee MDPI, Basel, Switzerland. This article is an open up admission article distributed under the terms and weather of the Creative Eatables Attribution (CC BY) license (https://creativecommons.org/licenses/past/4.0/).
Source: https://www.mdpi.com/2075-4418/11/5/888/htm
0 Response to "False Positive Results in Fetal Genetic Testing Peer Reviewed"
Enviar um comentário